
Source: https://www.bbc.co.uk/news/articles/c1ddk1ddme5o
Continuous glucose monitors on the rise after FDA approval
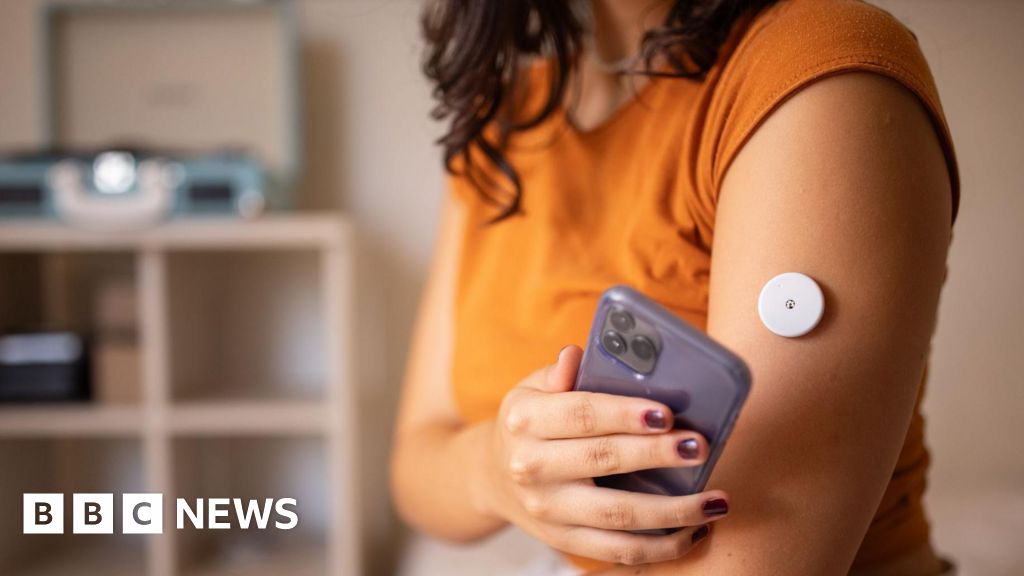
The use of continuous glucose monitors (CGMs) is expected to increase significantly following the US Food and Drug Administration (FDA) approval of the Freestyle Libre 3, the first CGM that does not require finger-prick calibration. This approval means that people with diabetes can now monitor their glucose levels continuously and receive real-time alerts on their smartphones without needing to prick their fingers for blood samples. The FDA's decision is considered a major breakthrough in diabetes management, with the potential to improve patient outcomes and reduce healthcare costs. The Freestyle Libre 3 is a small sensor that is worn on the back of the upper arm, and it transmits glucose readings every minute to a smartphone or other compatible device. The device is designed to be user-friendly and convenient, and it can help people with diabetes better manage their condition and avoid potentially dangerous high or low blood sugar levels. The approval of the Freestyle Libre 3 is expected to lead to increased adoption of CGMs, which could have a significant impact on the diabetes care landscape. More people may be able to access this technology, and it could lead to improvements in diabetes management and patient outcomes.
Summary
"The FDA's approval of the Freestyle Libre 3, a finger-prick-free continuous glucose monitor, is expected to significantly increase the use of CGMs. This breakthrough technology allows individuals with diabetes to monitor their glucose levels continuously and receive real-time alerts on their smartphones, potentially improving patient outcomes and reducing healthcare costs."
Updated at: 06.16.2024
Categories




